Electrons
When lightning strikes, millions of volts of electricity light up the sky. Electricity is a vital form of energy that is used to perform numerous daily activities.

Atoms release electrons, which are generally in a high-energy state, in order to maintain an energy balance. How is electricity produced? Atoms are made up of tiny particles called protons, neutrons, and electrons. Protons and neutrons are found in the atom's nucleus, while the electrons revolve around it. In most atoms, the number of protons equals the number of electrons.
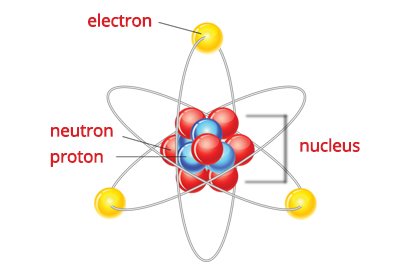
An atom is electrically neutral in this state. However, there are times when atoms lose electrons, particularly when they are exposed to an external energy source. When this happens, atoms lose negative charges and become more positive. The opposite may also happen. Atoms can gain additional electrons and become more negative. When two atoms with opposite charges meet, they tend to pull each other. This is called electrostatic attraction. When atoms of the same charges meet, they tend to push each other away. This is known as electrostatic repulsion. The attraction-repulsion phenomenon brought about by the interaction of electrons creates an electric force. When charges move due to this force, electricity is produced.
